
Over 16,545,290 people are on fubar.
What are you waiting for?

 Get ready for some real pew pew, because Northrop Grumman has just announced that the first ever high-energy, solid-state lethal laser for actual war applications is now available for ordering. This means that you can pay now a few millions and get yours for Xmas. The new Firestrike units offer 15 kilowatts of power, but can be combined to offer 100 kilowatts of technological terror, capable of actually destroying the enemy. According to the company, this new laser "changes the game" of military engagement. Nobody would say by judging the neutral looks of it:
Get ready for some real pew pew, because Northrop Grumman has just announced that the first ever high-energy, solid-state lethal laser for actual war applications is now available for ordering. This means that you can pay now a few millions and get yours for Xmas. The new Firestrike units offer 15 kilowatts of power, but can be combined to offer 100 kilowatts of technological terror, capable of actually destroying the enemy. According to the company, this new laser "changes the game" of military engagement. Nobody would say by judging the neutral looks of it:
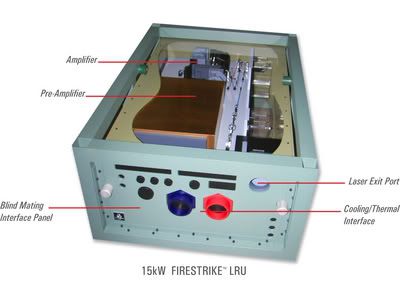 The FIRESTRIKE(tm) laser is a line replaceable system that allows for scaling a laser weapon to desired power levels for specific warfighting applications and platforms. Northrop Grumman believes that FIRESTRIKE(tm) laser will form the backbone of future laser weapon systems.
You can put together up to eight of these to achieve the maximum power available, although that would so much electric power that the military would have to mount them in tanks. Which, mind you, I bet it's exactly what they have in mind.
This is a big leap in laser weapons because, until now, the only effective way to get laser weapons to work with enough lethal power was using chemicals. These were extremely heavy and the whole firing process extremely hazardous. The solid-state laser in Firestrike is hard and very easy to manage, as it only requires electricity and has no by-products. It's also very rugged, according to Northrop's beam-cannon chief Dan Wildt:
This is a rugged electric laser with power levels, beam quality and runtime suitable for offensive and defensive military utility. Also available is a newly designed laser current source assembly (LCSA), which is compact, and specifically developed to precisely meet FIRESTRIKE(tm)'s power needs. Combined with advanced electro optical and/or infrared sensors, the FIRESTRIKE(tm) laser can provide self-defense, precision strike and enhanced situational awareness capabilities.
The lasers don't have to run intermittently either. There's no pew pew here. Just one long buzz that will last until it runs out of energy or coolant.
FIRESTRIKE(tm) Laser Features
Power: 15kW laser
Beam Quality: Nominally 1.5 times the diffraction limit
Size: Laser head - 12" x 23" x 40" (width, depth, height), Current source - 9" x 13" x 30"
Runtime: Continuous, as long as power and coolant are provided
Instant Turn-on: Zero to full power in less than 1/2 second
Safety_ Remote operation, customer interlock access, internal safety sensors
Control: Common Command and Control (C2) systems and Ethernet interfaces Low Power Setting Provides nominally 100 watt alignment beam Weight: 400 lbs per LRU
Ruggedization: Hardened LRUs with compact SSL technology engineered for mobility and field operations
The FIRESTRIKE(tm) laser is a line replaceable system that allows for scaling a laser weapon to desired power levels for specific warfighting applications and platforms. Northrop Grumman believes that FIRESTRIKE(tm) laser will form the backbone of future laser weapon systems.
You can put together up to eight of these to achieve the maximum power available, although that would so much electric power that the military would have to mount them in tanks. Which, mind you, I bet it's exactly what they have in mind.
This is a big leap in laser weapons because, until now, the only effective way to get laser weapons to work with enough lethal power was using chemicals. These were extremely heavy and the whole firing process extremely hazardous. The solid-state laser in Firestrike is hard and very easy to manage, as it only requires electricity and has no by-products. It's also very rugged, according to Northrop's beam-cannon chief Dan Wildt:
This is a rugged electric laser with power levels, beam quality and runtime suitable for offensive and defensive military utility. Also available is a newly designed laser current source assembly (LCSA), which is compact, and specifically developed to precisely meet FIRESTRIKE(tm)'s power needs. Combined with advanced electro optical and/or infrared sensors, the FIRESTRIKE(tm) laser can provide self-defense, precision strike and enhanced situational awareness capabilities.
The lasers don't have to run intermittently either. There's no pew pew here. Just one long buzz that will last until it runs out of energy or coolant.
FIRESTRIKE(tm) Laser Features
Power: 15kW laser
Beam Quality: Nominally 1.5 times the diffraction limit
Size: Laser head - 12" x 23" x 40" (width, depth, height), Current source - 9" x 13" x 30"
Runtime: Continuous, as long as power and coolant are provided
Instant Turn-on: Zero to full power in less than 1/2 second
Safety_ Remote operation, customer interlock access, internal safety sensors
Control: Common Command and Control (C2) systems and Ethernet interfaces Low Power Setting Provides nominally 100 watt alignment beam Weight: 400 lbs per LRU
Ruggedization: Hardened LRUs with compact SSL technology engineered for mobility and field operations global warming, polar bear lifevest, polar bear life preserver, environmental art, social commentary, green design, sustainable design, wildlife preservation
As the climate crisis mounts and Arctic icebergs slip away, polar bears are suffering starvation, population declines, and drowning as they must swim further and further to find food. Seeking to raise awareness for the endangered species’ plight, ADDI Concepts has taken wildlife preservation literally by designing a life-vest for displaced polar bears struggling to stay afloat as their homes sink into the sea.
global warming, polar bear lifevest, polar bear life preserver, environmental art, social commentary, green design, sustainable design, wildlife preservation
As the climate crisis mounts and Arctic icebergs slip away, polar bears are suffering starvation, population declines, and drowning as they must swim further and further to find food. Seeking to raise awareness for the endangered species’ plight, ADDI Concepts has taken wildlife preservation literally by designing a life-vest for displaced polar bears struggling to stay afloat as their homes sink into the sea.
 global warming, polar bear lifevest, polar bear life preserver, environmental art, social commentary, green design, sustainable design, wildlife preservation
Polar bears are facing a bleak future as Arctic icebergs continue to melt and ancient shelfs of ice collapse. The species inhabits only the Arctic Ocean and its surrounding areas, and they and can hunt consistently only from sea ice. ADDI Concepts conceived of their polar bear life jackets not as a solution for the endangered species, but as a means to increase awareness about global warming and inspire action. Their portfolio states: “A dog who lives most of its days carried around in an expensive handbag doesn’t need a camouflage hoodie and a small cap over its ears. There are a few other [creatures] who we should give at least the same attention”
The design group has also conceived of a bulletproof vest for Bengal tigers, whose numbers have decreased by 95% since 1910 due to illegal hunting.
+ ADDI Concepts
Via Dvice
global warming, polar bear lifevest, polar bear life preserver, environmental art, social commentary, green design, sustainable design, wildlife preservation
Polar bears are facing a bleak future as Arctic icebergs continue to melt and ancient shelfs of ice collapse. The species inhabits only the Arctic Ocean and its surrounding areas, and they and can hunt consistently only from sea ice. ADDI Concepts conceived of their polar bear life jackets not as a solution for the endangered species, but as a means to increase awareness about global warming and inspire action. Their portfolio states: “A dog who lives most of its days carried around in an expensive handbag doesn’t need a camouflage hoodie and a small cap over its ears. There are a few other [creatures] who we should give at least the same attention”
The design group has also conceived of a bulletproof vest for Bengal tigers, whose numbers have decreased by 95% since 1910 due to illegal hunting.
+ ADDI Concepts
Via Dvice

 Incoming president Barack Obama must decide the shuttle's fate soon if he wants to keeps its replacement on schedule, the Government Accountability Office says (Image: NASA)
Incoming president Barack Obama must decide the shuttle's fate soon if he wants to keeps its replacement on schedule, the Government Accountability Office says (Image: NASA)
US president-elect Barack Obama will need to decide soon whether to retire the space shuttle in 2010 or extend its life, a government oversight office said on Thursday.
The space shuttle is one of 13 'urgent' issues that face the next US president, according to a US Government Accountability Office (GAO) list. "These are issues that will require the attention of the President and Congress early on in the next administration," says GAO spokesperson Chuck Young.
Deciding the fate of the shuttle is particularly time-sensitive, Young says. If the government decides to fly more shuttle missions, it could impact how quickly NASA can move forward with a shuttle replacement, set to be ready to fly by March 2015.
The replacement, the centrepiece of a NASA programme called Constellation, would end a five-year gap in the US's ability to transport astronauts to space. During the interim, astronauts will have to hitch rides to the International Space Station on Russian Soyuz capsules.
Interdependent programmes
Extending the shuttle's lifetime means that if "NASA's budget doesn't change, it will put Constellation off", says Cristina Chaplain of the GAO.
But even with more money, NASA may not be able to close the gap in its access to space. That's because the shuttle and Constellation programmes are interdependent, Chaplain told New Scientist.
The agency needs to free up facilities and personnel that currently maintain the shuttle fleet for work on the replacement vehicle, an Apollo-inspired capsule called Orion that will launch atop the Ares I rocket.
Congress built in time for Obama to decide the shuttle's fate. NASA is not allowed to take any actions before 30 April 2009 that would prevent the shuttle from flying safely after its scheduled retirement in 2010, according to the agency's new authorisation act, which passed in October.
High costs
The agency has estimated that $2.5 billion to $4 billion a year will be needed to keep the shuttle flying past 2010. But some argue that the shuttle will need a thorough review if it is to fly much longer than that.
Technical issues with the shuttle replacement programme could also affect the decision about when to retire the shuttles. Some say the Ares I rocket may be prone to vibrations that could jolt astronauts.
Resolving Ares design issues could require as much as $7 billion more in order to keep the rocket on target for its first flight in March 2015, the Congressional Budget Office said on Monday.
Incoming president Barack Obama must decide the shuttle's fate soon if he wants to keeps its replacement on schedule, the Government Accountability Office says (Image: NASA)
Incoming president Barack Obama must decide the shuttle's fate soon if he wants to keeps its replacement on schedule, the Government Accountability Office says (Image: NASA)
US president-elect Barack Obama will need to decide soon whether to retire the space shuttle in 2010 or extend its life, a government oversight office said on Thursday.
The space shuttle is one of 13 'urgent' issues that face the next US president, according to a US Government Accountability Office (GAO) list. "These are issues that will require the attention of the President and Congress early on in the next administration," says GAO spokesperson Chuck Young.
Deciding the fate of the shuttle is particularly time-sensitive, Young says. If the government decides to fly more shuttle missions, it could impact how quickly NASA can move forward with a shuttle replacement, set to be ready to fly by March 2015.
The replacement, the centrepiece of a NASA programme called Constellation, would end a five-year gap in the US's ability to transport astronauts to space. During the interim, astronauts will have to hitch rides to the International Space Station on Russian Soyuz capsules.
Interdependent programmes
Extending the shuttle's lifetime means that if "NASA's budget doesn't change, it will put Constellation off", says Cristina Chaplain of the GAO.
But even with more money, NASA may not be able to close the gap in its access to space. That's because the shuttle and Constellation programmes are interdependent, Chaplain told New Scientist.
The agency needs to free up facilities and personnel that currently maintain the shuttle fleet for work on the replacement vehicle, an Apollo-inspired capsule called Orion that will launch atop the Ares I rocket.
Congress built in time for Obama to decide the shuttle's fate. NASA is not allowed to take any actions before 30 April 2009 that would prevent the shuttle from flying safely after its scheduled retirement in 2010, according to the agency's new authorisation act, which passed in October.
High costs
The agency has estimated that $2.5 billion to $4 billion a year will be needed to keep the shuttle flying past 2010. But some argue that the shuttle will need a thorough review if it is to fly much longer than that.
Technical issues with the shuttle replacement programme could also affect the decision about when to retire the shuttles. Some say the Ares I rocket may be prone to vibrations that could jolt astronauts.
Resolving Ares design issues could require as much as $7 billion more in order to keep the rocket on target for its first flight in March 2015, the Congressional Budget Office said on Monday.